Pharmaceutical Roots: Metformin – could leading antidiabetic become ‘The aspirin of the 21st century?’
Pharmaceutical Roots is a content series from LGC Mikromol investigating and outlining the natural origins of pharmaceutical substances, and offering a deeper dive into their uses, risks, and mechanisms of action.

Introduction
Taken orally, metformin hydrochloride is a leading biguanidine antihyperglycemic agent that manages high blood sugar levels in Type-2 diabetes. But despite its current status as the world’s most-prescribed glucose-lowering medicine, metformin has a less than straightforward history. One expert described the drug’s story as “turbulent”, adding that it has been “discovered, forgotten, rediscovered, re-purposed, rejected, rescued (and) exonerated” in the less than 100 years since it was first discovered. Here we tell that story – from metformin’s roots as a foul-smelling plant extract to its gradual emergence over the last century, and finally its potential therapeutic applications for the future.
History
Metformin and related biguanide compounds are derived from Galega officinalis – a bushy perennial plant also known as Goat’s rue, and found in temperate grassland regions of Asia and Europe. Employed for centuries to relieve symptoms ofdiabetes mellitus (formerly known as ‘sweet urine’), extracts of Goat’s rue containing the phytochemical guanidine have also been widely used as galactagogues to increase milk flow in both humans and animals. In 1653, Culpeper’s Complete Herbal listed g. officinalis as a remedy for worms, falling sickness (epilepsy), fever and pestilence, whileit also has proven weight loss and anticoagulant properties. First synthesised in the late 1870s, guanidine was found to reduce blood glucose when injected into rabbits in 1918. Subsequently, guanidine derivates – such as the monoguanidine, galegine, and the more potent diguanidine, synthalin - were introduced as potential diabetes cures. However, toxicity was soon observed, and by the 1930s they had been eclipsed by insulin, which had been discovered in 1922 and was starting to be produced on a larger scale.
As early as 1929, metformin had been shown to lower blood glucose in rabbits and dogs , and was also the least toxic of the various methyl biguanides tested at that time. However, according to diabetes expert Professor Cliff Bailey, “the real potential of these agents was underappreciated due to the high doses required to achieve modest glucose lowering in non-diabetic animals (compared with subsequent evidence in diabetic models).” This meant that biguanides including metformin were overlooked until the mid-1940s, when animal studies during the development of the guanidine-based antimalarial proguanil (paludrine) again demonstrated their ability to lower glucose levels. The search for more antimalarials resulted in proguanil being modified to metformin, which Filipino scientist Eusebio Garcia used successfully during a local cerebral influenza outbreak in 1949 and called flumamine.
Almost another decade passed until French physician Jean Sterne carried out the first full-scale programme of research into the pharmacodynamics of several guanidine-based compounds. Sterne singled out metformin as a particularly promising diabetic drug candidate, based on its proven glucose-lowering efficiency and lack of side-effects in animals, as well as the flumamine episode. Aron adopted his suggestion that metformin should be marketed under the brand name ‘Glucophage’ (or ‘glucose-eater’), and the new drug gained an initial foothold in the UK and Canada – although it was not introduced into the USA and used infrequently in Europe.
Part of the reason for metformin’s continued struggle to gain acceptance was that it was “tarnished” by association with phenformin and buformin – two fellow glucose-lowering guanidine derivates that initially became highly successful drugs, but were later withdrawn because of severe side-effects. Phenformin, the phenethyl biguanide relative of metformin, had gained global popularity as an alternative to sulphonylureas, especially in the USA, while the German-researched and marketed buformin became available across continental Europe. However, according to Bailey, “The risk of lactic acidosis, especially with phenformin and buformin was evident from the outset”, and ultimately forced the US Food and Drug Administration (FDA) to withdraw phenformin from the American market in 1978. Today, both phenformin and buformin are only available in a few countries around the world.
With a much lower incidence of lactic acidosis amongst metformin users, the drug’s time had almost come – but due to the phenformin affair, it first had to pass an intensive assessment process to gain marketing approval in the US. The drug’s owner, LIPHA pharmaceuticals, had to answer “an avalanche of questions from the FDA” after it bid for approval in 1986, but metformin ultimately launched in the US in 1995. After 2000, it became available in extended release form, and subsequently as a combination product with many other anti-diabetic agents. By 2020, it was the third most-prescribed medication in the US, as well as a fixture on the World Health Organisation’s List of Essential Medicines.
Mechanism of action
Metformin’s molecular mechanism is not completely understood, although several potential mechanisms of action have been proposed, including: inhibition of the mitochondrial respiratory chain, activation of AMP-activated protein kinase, and inhibition of glucagon-induced elevation of the cyclic adenosine monophosphate with reduced activation of protein kinase A. Metformin also exerts an anorexiant effect in most people by increased GDF15 secretion, reducing appetite and caloric intake.
Metformin decreases gluconeogenesis (glucose production) in the liver. It also exhibits an insulin-sensitising effect through inhibition of basal secretion from the pituitary gland, of growth hormone, adrenocorticotropic hormone, follicle stimulating hormone and expression of proopiomelanocortin. The average patient with Type-2 diabetes has three times the normal rate of gluconeogenesis, but metformin treatment reduces this by a third. AMPK activation is imperative in metformin’s ability to inhibit glucose production. AMPK is an important enzyme in insulin signalling in the body and the metabolism of glucose, although it is unknown how metformin increases the activity of AMPK. However, metformin does increase the concentration of AMP, which could activate AMPK allosterically at high levels. A newer theory involves binding to PEN-2.
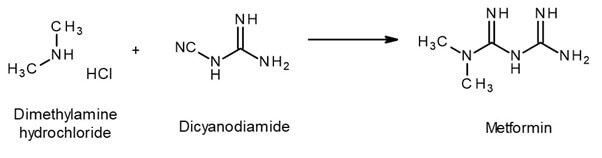
Synthesis
Outlook
While it has proved its efficacy in treating Type-2 diabetes, many current researchers are excited about metformin’s potential to treat other conditions – its versatility prompting the authors of one review article to dub it “the aspirin of the 21st century.”
The drug is already commonly prescribed ‘off label’ to treat Polycystic Ovary Syndrome (PCOS) because of its ability to increase menstrual regularity and reduce the obesity experienced by up to 80% of patients. There is also a “substantial literature” noting metformin’s beneficial effects on the macro- and micro-vasculature, as well as survey evidence that it can reduce heart disease independently from its glucose-lowering effects.
The number of clinical trials designed to assess the potential benefits of metformin in cancer treatment has, meanwhile increased from around 100 in 2013 to almost 400 in May 2022 – encompassing endometrial and ovarian, head and neck squamous cell, multiple myeloma, and thyroid cancers, plus lymphocytic leukaemia. Given that research has identified a link between elevated glucose levels and the risk of dementia, some scientists also believe metformin could potentially “offset the pathophysiology of Alzheimer's disease and arguably other neurodegenerative diseases.”
Your metformin analysis covered - with LGC Mikromol, Dr Ehrenstorfer, and TRC
To support your analysis and help ensure the accuracy of your quality control processes, LGC Mikromol supplies an ISO 17025-accredited pharmaceutical reference standard for metformin, together with a range of impurity products. You can also explore our full range of Mikromol API, impurity and excipient reference standards here.
We can also provide a large selection of TRC research chemicals to support your metformin studies, while LGC Dr Ehrenstorfer food, beverage and environment reference materials for metformin can facilitate your testing of its growing impact on the natural world.
|
