New ‘double-headed’ drug activates cancer cell death pathways

A new drug compound that rewires cancer driver circuitry to destroy cancer cells could have important applications for treating the disease, as well as other gene-regulated illnesses.
Stanford University scientists have developed a first-in-class drug that ‘rewires’ cancer drivers to kill cancer cells.
The TCIP1 molecule switches a transcription factor from a repressor of gene expression to an activator– unblocking the apoptosis cell death process that destroys cancers.
Reviewing the discovery in Nature, independent scientists said that the discovery not only represents ‘a fresh strategy’ for designing drugs to combat a range of cancers, but could also have applications for treating other gene-regulated diseases.
In designing TCIP1, the Stanford group leveraged the role of transcription factor B cell lymphoma 6 (BCL6) – a protein that binds to the promoters of apoptosis genes and suppresses their expression in cancers such as diffuse large B cell lymphoma (DLBCL).
Using chemically induced proximity (CIP) principles, they then synthesised a compound with a molecule that connected to both BCL6 and BRD4 – a transcriptional activator protein that usually promotes rapid growth in cancer cells. However, when linked to BCL6, BRD4 activates whatever BCL6 binds to, and turns on any gene expression it attempts to suppress – including apoptosis.
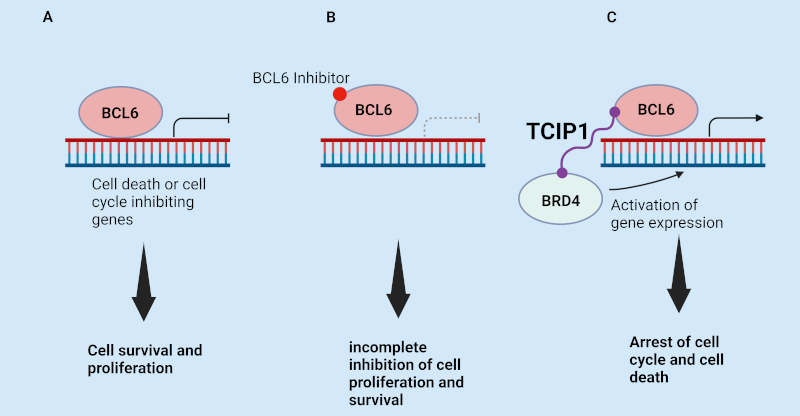
After carrying out tests on DLBCL cell lines, the group found that the novel molecule “increases binding of BRD4 by 50% over genomic BCL6-binding sites… (and) kills diffuse large B cell lymphoma cell lines, including chemotherapy-resistant, TP53-mutant lines… in 72 h.” It also upregulated the expression of hundreds of genes, many of which are targeted by BCL6 – including FOXO3 – “a master pro-apoptotic gene with a BCL6-binding site at its promoter.” Conversely, the new drug reduced the expression of genes such as MYC, whose activation is the hallmark of the initiation and maintenance of many cancers, in particular DLBCLs.
Also encouragingly, the new drug worked in a manner that avoided side-effects when tested on laboratory mice. Because BCL6 is also critical to the proper functioning of the lymphatic system, and BRD4 important to genome function, TCIP1 could potentially have negatively impacted off-target healthy tissues. However, while it caused “dramatic transcriptomic changes” in the animals’ spleens, adverse effects such as changes in mouse body weight and inflammation were not detected.
RNA sequencing studies also showed that “TCIPs produce their effect by activating cell death signalling and rewiring only a fraction of the cancer driver molecules per cell to drive the phenotype” - with 10 nM of TCIP1 producing only a roughly 1.5-fold increase in BRD4 at BCL6 sites over the genome, despite robust gene activation and cell killing. This data, the Stanford group argued, “support(s) the cellular evidence that TCIP1 acts in a context-specific manner dependent on coincident expression of BRD4 and BCL6”, and interfering little with regular activity elsewhere.
Presenting their findings in Nature, the Stanford group argued that “by making use of the intrinsic driving pathways of the cancer cell and rewiring them to activate pathways of cell death, we have introduced an approach to cancer chemotherapy that is analogous to a dominant, gain-of-function mutation in genetics.”
They also predicted that it would be possible to “generalize this strategy of killing cancer cells by rewiring the cancer driver circuitry” in other types of the disease, while the review of the findings identified BCL6-expressing follicular lymphoma, Burkitt lymphoma, and angioimmunoblastic T cell lymphoma as potential future targets for TCIP1.
Finally, the Stanford team speculated that the activation of genes by small-molecule TCIPs could have additional applications in other clinical disciplines – including activating death pathways in senescent cells, in immunotherapy, and in synthetic biology.
LGC Standards – for all your research chemical and drug discovery needs
LGC standards’ TRC brand has more than 40 years’ experience working through some of the most complex synthetic pathways to deliver you high quality research chemicals. We have a large portfolio of APIs, bioactive molecules and SILS, as well as synthetic chemistry tools such as building blocks and linkers.
Our TRC range contains many unique molecules for cancer research and drug discovery, including modulators of apoptosis and autophagy such as TRADD, BCL-2 and BAK.
And, if you can’t find the chemical you need, our skilled team of synthetic chemics would relish the challenge of designing it for you! Email info.trc@lgcgroup.com for more details.
We also offer an unparalleled collection of in vitro tools for cancer research from the world’s largest biorepository, ATCC – a number of which were used during Stanford’s work on TCIP1.
Our range includes >80 brain derived cell lines, neural progenitor cells, as well as 275 patient-derived models from the Human Cancer Models Initiative (HCMI) - such as organoids, neurospheres and suspension lines, as well as primary cells.
|
