Carprofen 1,3-Butylene Glycol Esters (Mixture of Regio- and Stereoisomers)
Product Code
MM1484.05-0025
Product Format
Neat
Molecular Formula
2 C19 H20 Cl N O3
Molecular Weight
691.64
Product Categories
Impurity Reference Materials , Non-steroidal anti-inflammatory drugs (NSAIDs), Mikromol
Product Type
ImpurityDrug Type
Analgesics
Share product
- Product Name: Carprofen 1,3-Butylene Glycol Esters (Mixture of Regio- and Stereoisomers)
- Product Code: MM1484.05-0025
- Brand: Mikromol
Product Information
Analyte Data
Analyte Name
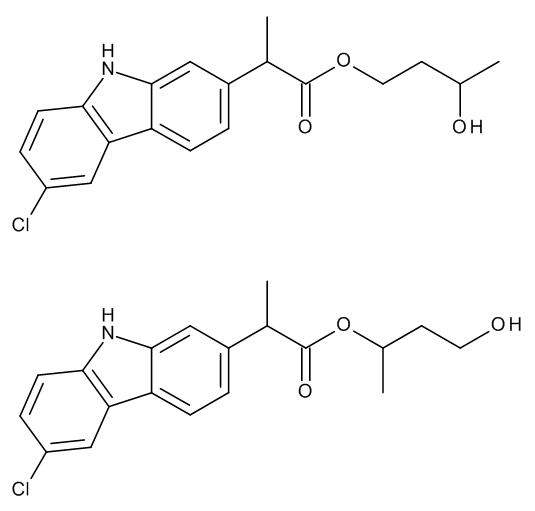
Carprofen 1,3-Butylene Glycol Esters (Mixture of Regio- and Stereoisomers)
Molecular Formula
2 C19 H20 Cl N O3
Molecular Weight
691.64
Accurate Mass
690.2263
SMILES
CC(O)CCOC(=O)C(C)c1ccc2c(c1)[nH]c3ccc(Cl)cc23.CC(CCO)OC(=O)C(C)c4ccc5c(c4)[nH]c6ccc(Cl)cc56
InChI
InChI=1S/2C19H20ClNO3/c1-11(22)7-8-24-19(23)12(2)13-3-5-15-16-10-14(20)4-6-17(16)21-18(15)9-13;1-11(7-8-22)24-19(23)12(2)13-3-5-15-16-10-14(20)4-6-17(16)21-18(15)9-13/h2*3-6,9-12,21-22H,7-8H2,1-2H3
IUPAC
4-hydroxybutan-2-yl 2-(6-chloro-9H-carbazol-2-yl)propanoate;3-hydroxybutyl 2-(6-chloro-9H-carbazol-2-yl)propanoate
Product Data
Storage Temperature
+5°C
Shipping Temperature
Room Temperature
Country of Origin
GERMANY
Product Type
Impurity
Product Format
Neat
Impurity Type
Degradation product
{{title}}
Please login or register to add to your favourites
Or continue browsing without access to favourites or pricing
Please log in to view pricing and add to cart
Or continue browsing to see available rounds without pricing information
If you don't yet have an account, please create an account create an account
- Product Code:
{{ isMultiplePacksize(product) ? getSourcePart(product) : product.code }} {{ isMultiplePacksize(product) ? getSourcePart(product) : product.code }}
Controlled product
Login to view full product details
{{ addToCartData.mixPtRmWarning }}
Do you want to proceed?
{{ errors.first('requestQuoteForm.firstName') }}
{{ errors.first('requestQuoteForm.lastName') }}
{{ errors.first('requestQuoteForm.email') }}
{{ errors.first('requestQuoteForm.phoneCountryCode') }}
{{ errors.first('requestQuoteForm.phoneNumber') }}
{{ errors.first('requestQuoteForm.company') }}
{{ errors.first('requestQuoteForm.selectedShippingAddress') }}
{{ errors.first('requestQuoteForm.shippingCompanyName') }}
{{ errors.first('requestQuoteForm.shippingLine1') }}
{{ errors.first('requestQuoteForm.shippingCountry') }}
{{ errors.first('requestQuoteForm.shippingState') }}
{{ errors.first('requestQuoteForm.shippingCity') }}
{{ errors.first('requestQuoteForm.shippingPostCode') }}
{{ errors.first('requestQuoteForm.billingCompanyName') }}
{{ errors.first('requestQuoteForm.billingLine1') }}
{{ errors.first('requestQuoteForm.billingCountry') }}
{{ errors.first('requestQuoteForm.billingState') }}
{{ errors.first('requestQuoteForm.billingCity') }}
{{ errors.first('requestQuoteForm.billingPostCode') }}
{{ errors.first('requestQuoteForm.deliveryCountry') }}
{{ errors.first('requestQuoteForm.packSize') }}
{{ errors.first('requestQuoteForm.quantity') }}

