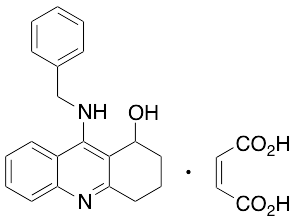
9-(Benzylamino)-1,2,3,4-tetrahydroacridin-1-ol Maleate
Product Code
TRC-B230000
CAS Number
Product Format
Neat
Molecular Formula
C20 H20 N2 O . C4 H4 O4
Molecular Weight
420.46
Product Categories
Share product
- Product Name: 9-(Benzylamino)-1,2,3,4-tetrahydroacridin-1-ol Maleate
- Product Code: TRC-B230000
- CAS Number: 113108-86-4
- Brand: TRC
Documentation
Looking for another lot?
To view all certificates of analysis immediately, please login to your account
or
Email download link
{{ errors.first('RequestCoaForm.lotNumber') }}
{{ errors.first('RequestCoaForm.requestEmail') }}
For information about our data processing activities, please visit our Privacy Notice.
Enter your email address and we'll email you the relevant CoA for lots:
{{ coaPopupData.packSize.coaSelectedLotNumbers }}
{{ errors.first('SendDownloadCoaLinkForm.coaEmail') }}
We will be sending the CoA to your email address {{ coaEmailPopupData.userEmail }}
Your request has been sent to our sales team to process.
Find an SDS for your region
{{ errors.first('RequestSdsForm.selectedRegion') }}
{{ errors.first('RequestSdsForm.sdsEmail') }}
For information about our data processing activities, please visit our Privacy Notice.
Your request has been sent to our sales team to process.
Product Information
Chemical Data
Analyte Name
9-(Benzylamino)-1,2,3,4-tetrahydroacridin-1-ol, Maleate
CAS Number
113108-86-4
Molecular Formula
C20 H20 N2 O . C4 H4 O4
Molecular Weight
420.46
Accurate Mass
420.1685
SMILES
OC1CCCc2nc3ccccc3c(NCc4ccccc4)c12.OC(=O)\C=C/C(=O)O
InChI
InChI=1S/C20H20N2O.C4H4O4/c23-18-12-6-11-17-19(18)20(15-9-4-5-10-16(15)22-17)21-13-14-7-2-1-3-8-14;5-3(6)1-2-4(7)8/h1-5,7-10,18,23H,6,11-13H2,(H,21,22);1-2H,(H,5,6)(H,7,8)/b;2-1-
IUPAC
9-(benzylamino)-1,2,3,4-tetrahydroacridin-1-ol;(Z)-but-2-enedioic acid
Product Data
Storage Temperature
-20°C
Shipping Temperature
Room Temperature
Country of Origin
CANADA
Product Format
Neat
Product Description
A potential Alzheimer's Disease therapeutic of low toxicity. Exhibits biochemical and pharmacological profile similar to THA except that it is far less toxic and without measurable liver toxicity in humans. Also showed potent in vitro inhibition of the uptake of radiolabeled noradrenaline and dopamine.
References: Shutske, G.M., et al.: J. Med. Chem., 32, 1805 (1989)
{{title}}
Please login or register to add to your favourites
Or continue browsing without access to favourites or pricing
Please log in to view pricing and add to cart
Or continue browsing to see available rounds without pricing information
If you don't yet have an account, please create an account create an account
- Product Code:
{{ isMultiplePacksize(product) ? getSourcePart(product) : product.code }} {{ isMultiplePacksize(product) ? getSourcePart(product) : product.code }}
Controlled product
Login to view full product details
{{ addToCartData.mixPtRmWarning }}
Do you want to proceed?
{{ errors.first('requestQuoteForm.firstName') }}
{{ errors.first('requestQuoteForm.lastName') }}
{{ errors.first('requestQuoteForm.email') }}
{{ errors.first('requestQuoteForm.phoneCountryCode') }}
{{ errors.first('requestQuoteForm.phoneNumber') }}
{{ errors.first('requestQuoteForm.company') }}
{{ errors.first('requestQuoteForm.selectedShippingAddress') }}
{{ errors.first('requestQuoteForm.shippingCompanyName') }}
{{ errors.first('requestQuoteForm.shippingLine1') }}
{{ errors.first('requestQuoteForm.shippingCountry') }}
{{ errors.first('requestQuoteForm.shippingState') }}
{{ errors.first('requestQuoteForm.shippingCity') }}
{{ errors.first('requestQuoteForm.shippingPostCode') }}
{{ errors.first('requestQuoteForm.billingCompanyName') }}
{{ errors.first('requestQuoteForm.billingLine1') }}
{{ errors.first('requestQuoteForm.billingCountry') }}
{{ errors.first('requestQuoteForm.billingState') }}
{{ errors.first('requestQuoteForm.billingCity') }}
{{ errors.first('requestQuoteForm.billingPostCode') }}
{{ errors.first('requestQuoteForm.deliveryCountry') }}
{{ errors.first('requestQuoteForm.packSize') }}
{{ errors.first('requestQuoteForm.quantity') }}
