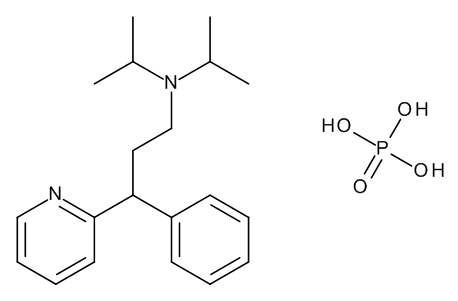
(3RS)-N,N-Bis(1-methylethyl)-3-phenyl-3-(pyridin-2-yl)propan-1-amine Dihydrogen Phosphate
Codice prodotto
MM0108.03-0025
Numero CAS
Descrizione EP
Disopyramide Impurity B as Dihydrogen Phosphate
Formato del prodotto
Neat
Formula molecolare
C20 H28 N2 . H3 O4 P
Peso molecolare
394.44
Categorie di prodotto
Cardiac drugs and beta blockers, Impurity Reference Materials , Mikromol
Tipologia prodotto
ImpurityTipo di farmaco
Antiarrhythmics
Share product
- Product Name: (3RS)-N,N-Bis(1-methylethyl)-3-phenyl-3-(pyridin-2-yl)propan-1-amine Dihydrogen Phosphate
- Codice prodotto: MM0108.03-0025
- Numero CAS: 2731375-73-6
- Marca: Mikromol
Informazioni sul prodotto
Dati analiti
Nome dell'analita
(3RS)-N,N-Bis(1-methylethyl)-3-phenyl-3-(pyridin-2-yl)propan-1-amine Dihydrogen Phosphate
Numero CAS
2731375-73-6
Formula molecolare
C20 H28 N2 . H3 O4 P
Peso molecolare
394.44
Massa accurata
394.2021
SMILES
CC(C)N(CCC(c1ccccc1)c2ccccn2)C(C)C.OP(=O)(O)O
InChI
InChI=1S/C20H28N2.H3O4P/c1-16(2)22(17(3)4)15-13-19(18-10-6-5-7-11-18)20-12-8-9-14-21-20;1-5(2,3)4/h5-12,14,16-17,19H,13,15H2,1-4H3;(H3,1,2,3,4)
IUPAC
3-phenyl-N,N-di(propan-2-yl)-3-pyridin-2-ylpropan-1-amine;phosphoric acid
Dati del prodotto
Temperatura di conservazione
+5°C
Temperatura di spedizione
Room Temperature
Paese di origine
GERMANY
Tipologia prodotto
Impurity
Formato del prodotto
Neat
Impurity Type
By-product
{{title}}
Effettua il login o registrati per aggiungere ai tuoi preferiti
Oppure continua a navigare senza accedere ai preferiti o ai prezzi
Effettua l'accesso per visualizzare i prezzi e aggiungere al carrello
Oppure continua a sfogliare per vedere i round disponibili senza le informazioni sui prezzi
- Codice prodotto:
{{ isMultiplePacksize(product) ? getSourcePart(product) : product.code }} {{ isMultiplePacksize(product) ? getSourcePart(product) : product.code }}
Controlled product
Login to view full product details
{{ addToCartData.mixPtRmWarning }}
Desideri procedere?
{{ errors.first('requestQuoteForm.firstName') }}
{{ errors.first('requestQuoteForm.lastName') }}
{{ errors.first('requestQuoteForm.email') }}
{{ errors.first('requestQuoteForm.phoneCountryCode') }}
{{ errors.first('requestQuoteForm.phoneNumber') }}
{{ errors.first('requestQuoteForm.company') }}
{{ errors.first('requestQuoteForm.selectedShippingAddress') }}
{{ errors.first('requestQuoteForm.shippingCompanyName') }}
{{ errors.first('requestQuoteForm.shippingLine1') }}
{{ errors.first('requestQuoteForm.shippingCountry') }}
{{ errors.first('requestQuoteForm.shippingState') }}
{{ errors.first('requestQuoteForm.shippingCity') }}
{{ errors.first('requestQuoteForm.shippingPostCode') }}
{{ errors.first('requestQuoteForm.billingCompanyName') }}
{{ errors.first('requestQuoteForm.billingLine1') }}
{{ errors.first('requestQuoteForm.billingCountry') }}
{{ errors.first('requestQuoteForm.billingState') }}
{{ errors.first('requestQuoteForm.billingCity') }}
{{ errors.first('requestQuoteForm.billingPostCode') }}
{{ errors.first('requestQuoteForm.deliveryCountry') }}
{{ errors.first('requestQuoteForm.packSize') }}
{{ errors.first('requestQuoteForm.quantity') }}

